Insights: A Cerus Leadership Blog
MAXIMIZING THE CLINICAL IMPACT OF PLATELET DONATIONS IN EXTRAORDINARY TIMESRichard J. Benjamin MD Ph.D. FRCPath, Chief Medical Officer at Cerus
Maximizing the Clinical Impact of Platelet Donations in Extraordinary Times
(August 2020) As we head into our fifth month of global lockdown, it’s crucial that we recognize the impact of the global pandemic on our society and healthcare system. In particular, COVID-19 has layered new and complex textures over our blood donation and transfusion practices, highlighting a unique opportunity to modify transfusion practices and expand the donor pool, avoid blood shortages and more effectively utilize the gift of blood donation. The current pandemic is the latest of many blood supply interruptions that have been as mundane as seasonal holidays or as life-threatening as COVID-19.
Platelet concentrate (PC) transfusions treat and prevent bleeding, and also enable life-giving therapies for cancer and other serious conditions. Their short shelf-life of 5 days results in irregular local shortages, as hospital demand fluctuates and the supply varies with the vagaries of donor availability. Shortages are exacerbated by recent changes in donor suitability requirements, such as the increase in the minimum hemoglobin requirement for men and policies leading to the deferral of ~ 20% of female donors in order to prevent transfusion-related acute lung injury (TRALI).
The implementation of bacterial risk mitigation strategies mandated by FDA guidance in March 2021 will further exacerbate shortages by increasing product losses to perform culture testing, as well as due to discards caused by frequent false-positive results.
A Path to Change
To address U.S. shortages, improve safety, and better platelet transfusion practice, Cerus has developed a pathogen reduction program called the INTERCEPT Blood System for Platelets, which reduces the risk of transfusion-transmitted infections. In concert with improved inventory management, rationalizing platelet dose and extension of shelf-life to seven days, these modifications can improve the safety and availability of PC transfusions, optimize donor management, as well as platelet dosing strategies, and ultimately improve patient outcomes.
The Impact of Donor Deferrals on our Blood Supply
PC in the U.S. are mostly derived from apheresis procedures performed on regular donors who donate the equivalent of one to three doses, as many as 24 times each year. Male donors with a low risk of causing TRALI are preferred and constitute the majority of this highly specialized donor pool. Due to bans on international travel or other pandemic-related disruptions, donor deferrals of even a few of these prolific donors can have a devastating effect on the PC supply.
The Revised Recommendations to Reduce the Risk of Transfusion-Transmitted Malaria published by FDA in April 2020, however, allows for the acceptance of donors otherwise deferred for risk of malaria due to travel to an endemic country when the resulting PC is treated with the INTERCEPT System. This dispensation from the FDA has a broad potential impact if applied in the future to other travel deferrals such as for emerging infectious diseases (e.g., SARS-CoV-2 and Zika virus), where donors who have traveled to high-risk areas may be deferred from donation.
The same rationale for utilizing INTERCEPT pathogen reduction to broaden the donor pool could be applied to men who have sex with men (MSM). Since males have a very low risk of carrying HLA-antibodies, they are ideal donors for PC products. The FDA has progressively recognized the potential for MSM blood donation, most recently with the publication in April 2020 of the Revised Recommendations for Reducing the Risk of Human Immunodeficiency Virus Transmission by Blood and Blood Products: Guidance for Industry. MSM donors may now donate blood with a three-month deferral from MSM activity. The availability of the INTERCEPT Blood System provides an additional layer of safety to protect patients from transfusion-transmitted infection. For blood collectors who use the INTERCEPT Blood System for Platelets, the FDA could consider approving the use of individual behavioral risk assessment independent of sexual orientation to replace the current duration-of-abstinence based approach to deferrals, addressing the major concerns for increased recipient risk from donations from this donor pool.
Platelet Dose and Why it Matters
The U.S. should also consider the advantages of reducing its apheresis PC minimum content standard to align with international practice (≥2.5 x 1011 platelets – a ~17% decrease from the U.S. standard), to increase the frequency with which multiple PCs can be collected in one donation. The current minimum apheresis PC content of ≥3.0 x1011/unit required by the U.S. FDA for quality control purposes is based on historical manufacturing standards and is not required for whole blood-derived PCs. This change might help to compensate for increased processing losses necessary to implement the mandated bacterial risk mitigation strategies.
The U.S. has among the highest PC per capita use in the world (~7.0 PCs per 1,000 population/year). The U.S. minimum apheresis PC content of ≥3.0 x1011 is also higher than it is in most other countries, with the European Directorate for the Quality of Medicines (EDQM) standard being >2.0 x1011. Most countries require at least 2.0-2.5 x 1011 platelets per unit. Lower PC content does not generally correlate with more platelet transfusions on a national scale. The combination of high per capita use and high content in the U.S. requires the frequent collection of large numbers of platelets from each donor, causing a strain on the supply chain, and exacerbating the risk of shortages. Indeed, the PLAtelet DOse (PLADO) study demonstrated that in hospitalized, hematology/oncology patients, the use of low platelet doses (~2.1×1011/bag for a 70 kg patient), resulted in the lower per-patient total number of platelets transfused than with higher doses. There was no impact on patient safety or on clinically significant bleeding, arguing for the safety of lowering the US minimum platelet content.
Achieving Seven-Day Storage
Because PCs have such a short shelf life, they often outdate and are wasted before they can be used, further constraining the supply. The effective hospital shelf life is reduced by current bacterial culture screening that requires a delay before release into inventory. Increasingly, regulatory agencies have recognized the wisdom of extended shelf-life of seven days storage, so long as the risk of bacterial sepsis is adequately mitigated. With the implementation of FDA Guidance in 2021, platelets that are screened by delayed large volume culture (DLVC) can be labeled for seven-day storage, but only if subjected to a 48-hour delay and large volume (16-20 mL) sampling of each final PC. Delayed sampling leads to frequent transfusion of older platelets (Figure 1). In contrast, many European hospitals routinely utilize INTERCEPT pathogen reduced PCs that may be released to hospital inventory more rapidly and can often be transfused on day three to five of storage (Figure 1). Real-world evidence in Switzerland shows strong evidence of safety and efficacy1. Though INTERCEPT pathogen-reduced PC with seven-day storage is not yet available in the U.S., Cerus is working to obtain FDA approval that will open the door to more effective inventory management and reduced wastage with improved availability.
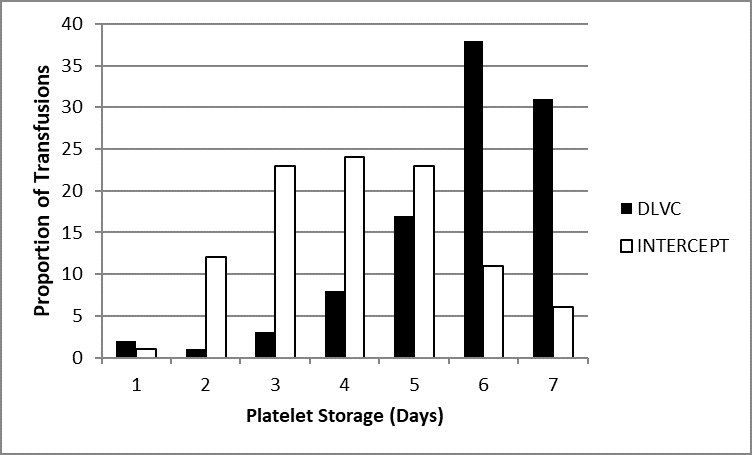
Figure 1: Age distribution at the time of transfusion of platelets stored for up to 7 days in Basel, Switzerland using the INTERCEPT Blood System for Platelets (INTERCEPT), or in Hamilton, Canada with delayed large volume culture (DLVC) screening.
Conclusions
The universal use of the INTERCEPT Blood System for Platelets, as is already implemented in France, Belgium, and Switzerland may meaningfully improve PC safety and availability here in the U.S. Donor deferral policies could be reassessed to minimize unnecessary deferrals and to expand the donor pool. Clinically, there is a strong rationale for reducing the U.S. minimum apheresis PC content requirement to align with international practice. With the potential of extended storage to seven days with effective bacterial safety measures, PC inventories may be rationalized to improve utilization and reduce potential outdates. With the INTERCEPT Blood System for Platelets, PC therapies may be optimized to minimize wastage while reducing the overall risk of bacterial infections as well as known and emerging viral infections.

About Dr. Richard Benjamin
Dr. Benjamin is responsible for Clinical Development and Medical Affairs at Cerus Corporation. He previously served as Chief Medical Officer of the American Red Cross. Dr. Benjamin is a Professor of Pathology at Georgetown University, Washington, D.C., and served as a board member and regional director for the ISBT. He serves as industry representative on the DHHS Secretary’s Advisory Committee on Blood and Tissue Safety and Availability. He was the medical director of the Adult Transfusion Service at the Joint Program in Transfusion Medicine at Harvard University. He received his medical training in Cape Town, South Africa, pathology training at the Brigham and Women’s Hospital in Boston, a Ph.D. at Cambridge University and performed post-doctoral research at Stanford University, CA.